Search Thermo Fisher Scientific
Thermo Scientific Chemicals
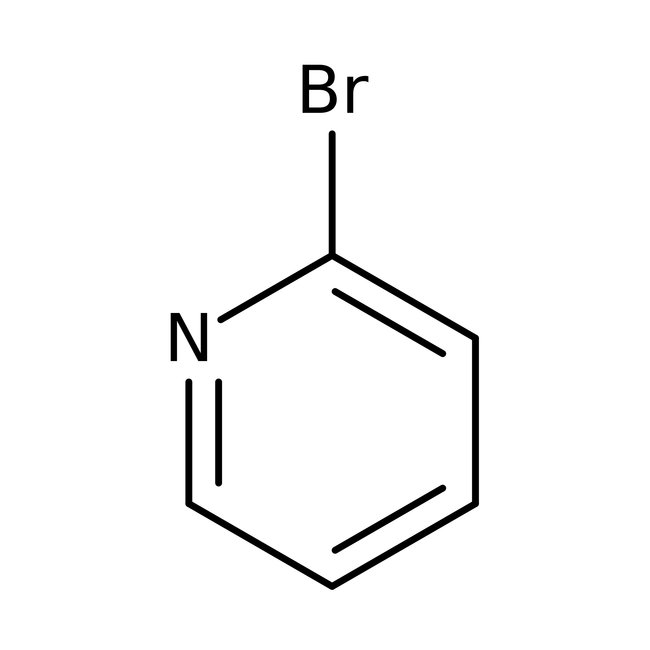
2-Bromopyridine, 99%
CAS: 109-04-6 | C5H4BrN | 157.998 g/mol
Catalog number ALFA13241.22
Price (MYR)
674.00
EA
Quantity:
100 g
Price (MYR)
674.00
EA
Specifications
Chemical Name or Material2-Bromopyridine
CAS109-04-6
Health Hazard 1H226-H301+H331-H310-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H310-H315-H319-H335
Toxic if swallowed.
Fatal in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H310-H315-H319-H335
Toxic if swallowed.
Fatal in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P233-P240-P241-P242-P243-P261-P262-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P305+P351+P338-P310-P311-P312-P330-P332+P313-P362-P374-P380-P501c
View more
2-Bromopyridine is used in the preparation of a variety of biologically active compounds like antimalarial agents. It is also used to prepare beta-adrenoceptor agonist.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromopyridine is used in the preparation of a variety of biologically active compounds like antimalarial agents. It is also used to prepare beta-adrenoceptor agonist.
Solubility
Slightly miscible with water.
Notes
Keep away from heat, sparks, and flame. Hygroscopic. Incompatible with strong oxidizing agents, strong acids and acid chlorides.
2-Bromopyridine is used in the preparation of a variety of biologically active compounds like antimalarial agents. It is also used to prepare beta-adrenoceptor agonist.
Solubility
Slightly miscible with water.
Notes
Keep away from heat, sparks, and flame. Hygroscopic. Incompatible with strong oxidizing agents, strong acids and acid chlorides.
RUO – Research Use Only
General References:
- Bromopyridines can be converted to the Grignards by Mg exchange with i-PrMgCl: Tetrahdron Lett., 40, 4339 (1999).
- Reaction with n-BuLi gives 2-lithio-pyridine. Subsequent reaction at low temperatures with PCl3 gives, as the major product, 2,2'-bipyridine, along with a low yield of the expected tris(2-pyridyl)phosphine. POCl3 or SOCl2 also promote the symmetrical coupling reaction: Heteroatom. Chem., 5, 409 (1994). In contrast, reaction with LDA in THF at -78° results in lithiation at the 3-position, giving access to 3-substituted 2-bromopyridines: Synthesis, 235, 237 (1982). See also: Org. Lett., 3, 835 (2001).
- Kathiravan, S.; Ghosh, S.; Hogarth, G.; Nicholls, I. A. Copper catalysed amidation of aryl halides through chelation assistance. Chem. Commun. 2015, 51 (23), 4834-4837.
- Liu, C.; Li, X.; Liu, C.; Wang, X.; Qiu, J. Palladium-catalyzed ligand-free and efficient Suzuki-Miyaura reaction of heteroaryl halides with MIDA boronates in water. RSC Adv. 2015, 5 (67), 54312-54315.