Search Thermo Fisher Scientific
Thermo Scientific Chemicals
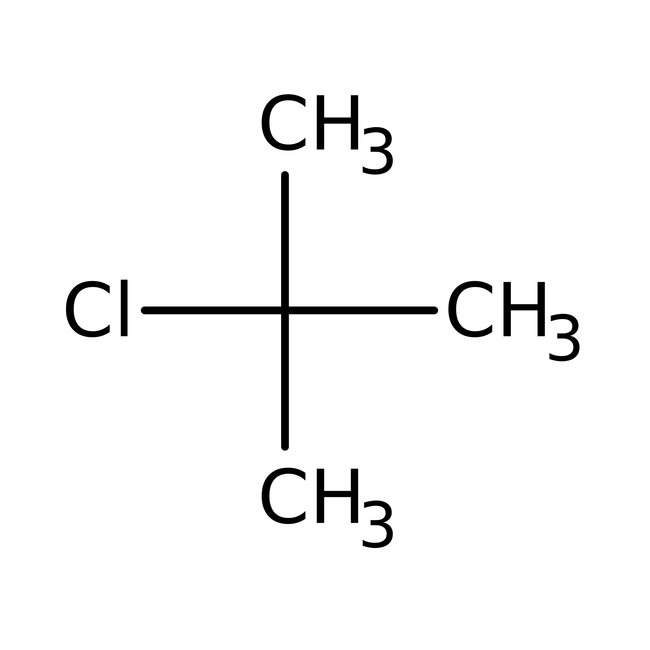
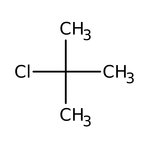
tert-Butyl chloride, 98+%
CAS: 507-20-0 | C4H9Cl | 92.566 g/mol
Catalog number ALFA13004.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or Materialtert-Butyl chloride
CAS507-20-0
Health Hazard 1H225
Health Hazard 2GHS H Statement
H225
Highly flammable liquid and vapour.
H225
Highly flammable liquid and vapour.
Health Hazard 3P210-P233-P240-P241-P242-P243-P280-P303+P361+P353-P370+P378q-P501c
View more
tert-Butyl chloride plays an important role as a starting material to perform nucleophilic substitution reactions in order to prepare alcohol and alkoxides salts. It is used as an alkylating agent for the introduction of tert-butyl group and is also involved in Friedel-Crafts reactions. It is employed as an intermediate for the synthesis of agrochemicals and pharmaceuticals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
tert-Butyl chloride plays an important role as a starting material to perform nucleophilic substitution reactions in order to prepare alcohol and alkoxides salts. It is used as an alkylating agent for the introduction of tert-butyl group and is also involved in Friedel-Crafts reactions. It is employed as an intermediate for the synthesis of agrochemicals and pharmaceuticals.
Solubility
Miscible with alcohol, benzene, chloroform and ether. Slightly miscible with water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and strong bases.
tert-Butyl chloride plays an important role as a starting material to perform nucleophilic substitution reactions in order to prepare alcohol and alkoxides salts. It is used as an alkylating agent for the introduction of tert-butyl group and is also involved in Friedel-Crafts reactions. It is employed as an intermediate for the synthesis of agrochemicals and pharmaceuticals.
Solubility
Miscible with alcohol, benzene, chloroform and ether. Slightly miscible with water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Stathi, P.; Deligiannakis, Y.; Avgouropoulos, G.; Louloudi, M. Efficient H 2 production from formic acid by a supported iron catalyst on silica. Appl. Catal., A 2015, 498, 176-184
- Sedov, I. A.; Stolov, M. A.;Solomonov, B. N. tert-Butyl chloride as a probe of the solvophobic effects. Fluid Phase Equilib. 2014, 382 164-168.