Search Thermo Fisher Scientific
Thermo Scientific Chemicals
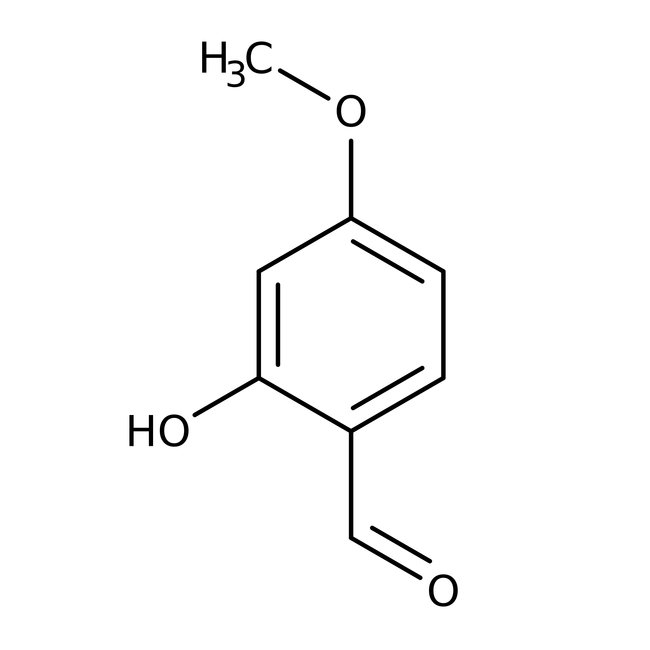
2-Hydroxy-4-methoxybenzaldehyde, 98%
CAS: 673-22-3 | C8H8O3 | 152.15 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12971.03 | 1 g |
Catalog number ALFA12971.03
Price (MYR)
250.00
EA
Quantity:
1 g
Price (MYR)
250.00
EA
Specifications
Chemical Name or Material2-Hydroxy-4-methoxybenzaldehyde
CAS673-22-3
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
It is employed in the synthesis of Schiff base ligand. It is applied as a reactant in the synthesis of LPA1R antagonists used in the inhibition of LPA-induced proliferation and contraction of normal human lung fibroblasts. Also used in the synthesis of tyrosine kinase 6 proteinase inhibitors.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is employed in the synthesis of Schiff base ligand. It is applied as a reactant in the synthesis of LPA1R antagonists used in the inhibition of LPA-induced proliferation and contraction of normal human lung fibroblasts. Also used in the synthesis of tyrosine kinase 6 proteinase inhibitors.
Solubility
Solubility in methanol is almost transparent. Insoluble in water.
Notes
Air sensitive. Store away from oxidizing agents, bases and air/oxygen. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Store under inert gas.
It is employed in the synthesis of Schiff base ligand. It is applied as a reactant in the synthesis of LPA1R antagonists used in the inhibition of LPA-induced proliferation and contraction of normal human lung fibroblasts. Also used in the synthesis of tyrosine kinase 6 proteinase inhibitors.
Solubility
Solubility in methanol is almost transparent. Insoluble in water.
Notes
Air sensitive. Store away from oxidizing agents, bases and air/oxygen. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Store under inert gas.
RUO – Research Use Only
General References:
- Basak S, et al. Synthesis, crystal structures and fluorescence properties of two new di-and polynuclear Cd (II) complexes with N2O donor set of a tridentate Schiff base ligand.Polyhedron.,2008,27(4), 1193-1200.
- Jihua Wang, et al. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxy-4-methoxybenzaldehyde.Molecules.,2010,15(8), 5807-5817 .
- I Kubo and I Kinst-Hori. 2-Hydroxy-4-methoxybenzaldehyde: a potent tyrosinase inhibitor from African medicinal plants.lanta Med.,1999,65(1), 19-22.
- Condensation with t-butyl acrylate results in cyclization to t-butyl 7-methoxychromene-3-carboxylate: J. Med. Chem., 36, 3580 (1993):
- With (Ethoxycarbonyl methyl ene) triphenyl phosphorane, A12896, in N,N-diethylaniline cyclization to 7-methoxycoumarin occurs: Heterocycles, 39, 613 (1994).