Search Thermo Fisher Scientific
Thermo Scientific Chemicals
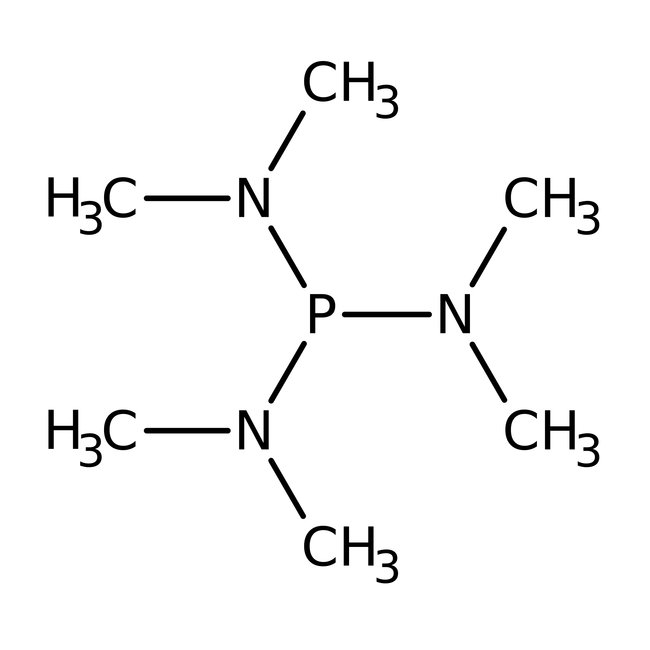
Hexamethylphosphorous triamide, 97%
CAS: 1608-26-0 | C6H18N3P | 163.21 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12571.06 | 5 g |
Catalog number ALFA12571.06
Price (MYR)
397.00
EA
Quantity:
5 g
Price (MYR)
397.00
EA
Specifications
Chemical Name or MaterialHexamethylphosphorous triamide
CAS1608-26-0
Health Hazard 1H226-H315-H319-H335-H351
Health Hazard 2GHS H Statement
H340-H350-H226
May cause genetic defects.
May cause cancer.
Flammable liquid and vapour.
H340-H350-H226
May cause genetic defects.
May cause cancer.
Flammable liquid and vapour.
Health Hazard 3P201-P202-P210-P233-P240-P243-P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P308+P313-P332+P313-P362-P374-P380-P501c
View more
Hexamethylphosphorous triamide is used as a reagent in organic synthesis as a phosphorylating agent. It is associated with carbon tetrachloride for the substitution of hydroxy groups with chlorides. It is involved in the preparation of epoxides and arene oxides from aldehydes and aryldialdehdyes respectively. It is used in the preparation of carbonates as well as reduction of ozonides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hexamethylphosphorous triamide is used as a reagent in organic synthesis as a phosphorylating agent. It is associated with carbon tetrachloride for the substitution of hydroxy groups with chlorides. It is involved in the preparation of epoxides and arene oxides from aldehydes and aryldialdehdyes respectively. It is used in the preparation of carbonates as well as reduction of ozonides.
Solubility
Miscible with water.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
Hexamethylphosphorous triamide is used as a reagent in organic synthesis as a phosphorylating agent. It is associated with carbon tetrachloride for the substitution of hydroxy groups with chlorides. It is involved in the preparation of epoxides and arene oxides from aldehydes and aryldialdehdyes respectively. It is used in the preparation of carbonates as well as reduction of ozonides.
Solubility
Miscible with water.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Reduces aromatic aldehydes to symmetrical epoxides in good yield: J. Am. Chem. Soc., 85, 1884 (1963). Both cis- and trans-isomers are formed. For list of examples, see: Org. Synth. Coll., 5, 358 (1973). Under suitable conditions, reaction of the intermediate with a second aldehyde can lead to mixed deoxybenzoins or diaryl enamines: Synthesis, 225 (1991).
- Has also been used for a variety of other reductions including that of ozonolysis intermediates: Helv. Chim. Acta, 50, 2387 (1967), and of primary alkyl nitro compounds to nitriles: Synthesis, 36 (1979). Bromohydrins can be converted to alkenes, by reductive elimination from their triflate esters: J. Am. Chem. Soc., 102, 1433 (1980):
- Reacts with BrCCl3 to give dichloromethylenephosphorane Cl2C=PPh3, which undergoes Wittig reaction with aldehydes to give 1,1-dichloroalkenes, giving better results than the CCl4/PPh3 combination: Tetrahedron Lett., 1237, 1239 (1977); Synthesis, 554 (1980). Similarly, with Br2CF2, the CF2 group can be transferred to both aldehydes and ketones: J. Fluorine Chem., 1, 123 (1971); Synth. Commun., 3, 197 (1973).
- Ioannou, P. V.; Tsivgoulis, G. M. The reduction of p-arsanilic acid (p-aminophenylarsonic acid) to its arsonous acid or arsine oxide: A case study. Main Group Chem. 2015, 14 (3), 237-253.
- Bhat, C.; Kumar, A. Synthesis of Allokainic Acid: A Review. Asian J. Org. Chem. 2015, 4 (2), 102-115.