Search Thermo Fisher Scientific
Thermo Scientific Chemicals
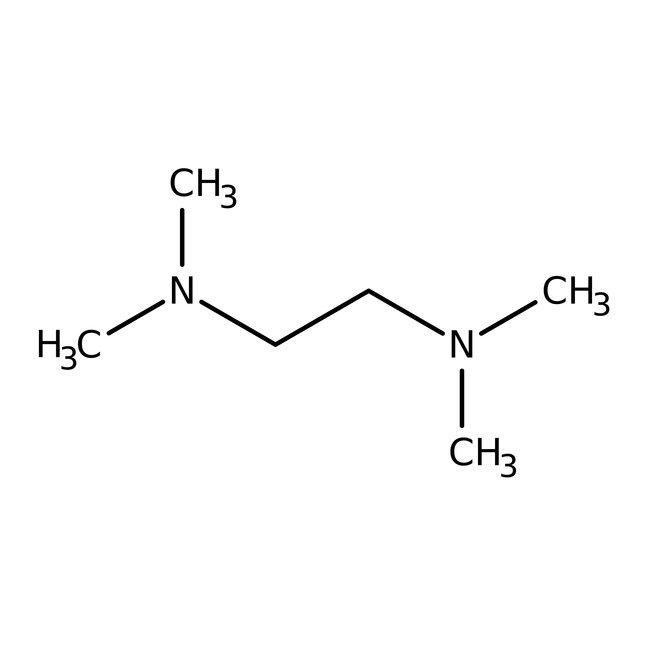
N,N,N',N'-Tetramethylethylenediamine, 99%
CAS: 110-18-9 | C6H16N2 | 116.208 g/mol
Catalog number ALFA12536.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or MaterialN,N,N',N'-Tetramethylethylenediamine
CAS110-18-9
Health Hazard 1H225-H302+H332-H314-H335
Health Hazard 2GHS H Statement
H225-H314-H302-H332
Highly flammable liquid and vapour.
Causes severe skin burns and eye damage.
Harmful if swallowed.
Harmful if inhaled.
H225-H314-H302-H332
Highly flammable liquid and vapour.
Causes severe skin burns and eye damage.
Harmful if swallowed.
Harmful if inhaled.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
N,N,N',N'-Tetramethylethylenediamine is used with ammonium persulfate to catalyze the polymerization of acrylamide and bis-acrylamide to get polyacrylamide gels. It is also used for the separation of proteins or nucleic acids. Further, it is employed as a ligand for metal ions like zinc and copper. It is actively involved in the formation of anionic organometallic complex.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N,N,N′,N′-Tetramethylethylenediamine is used with ammonium persulfate to catalyze the polymerization of acrylamide and bis-acrylamide to get polyacrylamide gels. It is also used for the separation of proteins or nucleic acids. Further, it is employed as a ligand for metal ions like zinc and copper. It is actively involved in the formation of anionic organometallic complex.
Solubility
Miscible with water.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, carbon dioxide and copper.
N,N,N′,N′-Tetramethylethylenediamine is used with ammonium persulfate to catalyze the polymerization of acrylamide and bis-acrylamide to get polyacrylamide gels. It is also used for the separation of proteins or nucleic acids. Further, it is employed as a ligand for metal ions like zinc and copper. It is actively involved in the formation of anionic organometallic complex.
Solubility
Miscible with water.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, carbon dioxide and copper.
RUO – Research Use Only
General References:
- Widely used complexing agent in lithiation reactions, often giving enhanced reactivity in otherwise difficult metallations: J. Am. Chem. Soc., 92, 4664 (1970). Subsequent work by Collum and others indicated that the situation may be much less straightforward than was previously thought, but the effect of TMEDA is likely to be most pronounced in the absence of strong donor solvents such as THF. For a discussion of the factors involved, see: Acc. Chem. Res., 25, 448 (1992). Under some conditions also, TMEDA itself may undergo lithiation: Organometallics, 13, 5173 (1994).
- Used in combination with alkyllithiums in the ortho-metallation of aromatics; see, e.g.: Org. Synth. Coll., 6, 478 (1988); Org. Synth. Coll., 9, 559 (1998). For discussion of the dramatic acceleration of the rate of ortho-lithiation of anisole in the presence of TMEDA, effective also in sub-stoichiometric amounts, see: Tetrahedron Lett., 35, 385 (1994). See also Tetrahedron Lett., 35, 401 (1994), J. Org. Chem., 62, 3024 (1997) for further discussion of the role of TMEDA in ortho-metallation.
- The oxidative coupling of terminal acetylenes to diynes, by O2 in the presence of CuCl, is improved by addition of TMEDA as a complexing agent: J. Org. Chem., 27, 3320 (1962). For example and discussion, see: Org. Synth. Coll., 8, 63 (1993):
- In catalytic amounts, produces up to a 20-fold increase in reaction rate in aqueous Michael additions catalyzed by ytterbium triflate: J. Org. Chem., 71, 352 (2006).