Search Thermo Fisher Scientific
Thermo Scientific Chemicals
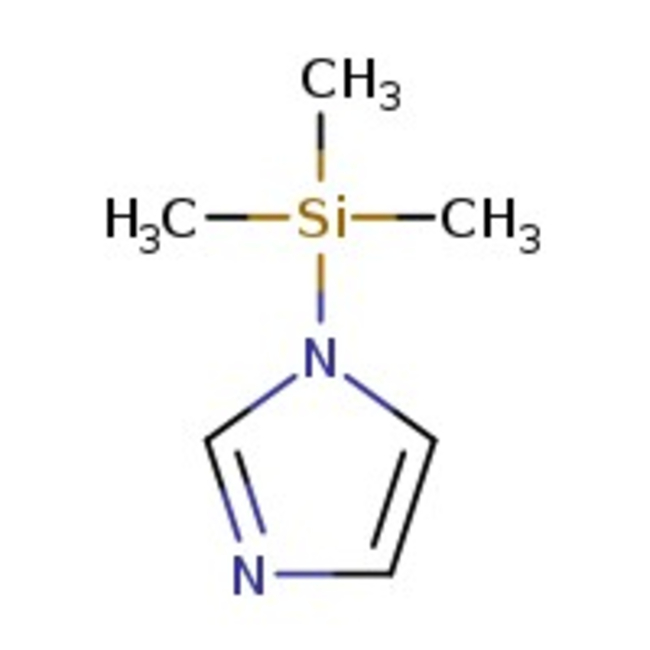
1-(Trimethylsilyl)imidazole, 97%
CAS: 18156-74-6 | C6H12N2Si | 140.26 g/mol
Catalog number ALFA12512.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material1-(Trimethylsilyl)imidazole
CAS18156-74-6
Health Hazard 1H225-H315-H319-H335
Health Hazard 2GHS H Statement
H225-H315-H319-H335
Highly flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H225-H315-H319-H335
Highly flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P312-P332+P313-P363-P370+P378q-P501c
View more
1-(Trimethylsilyl)imidazole acts as a silylating agent for alcohols, carbohydrates and 1,3-dicarbonyl compounds. It is a derivatization reagent used for selective silylation of hydroxyl groups and carboxylic acid without affecting the amino group. It employed as an intermediate for the synthesis of imidazole derivatives. It plays an essential role to synthesize polysubstituted chiral spirotetrahydropyrans. It is also used in the quantification of pintol after derivatization by gas chromatography/ mass spectrometry.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-(Trimethylsilyl)imidazole acts as a silylating agent for alcohols, carbohydrates and 1,3-dicarbonyl compounds. It is a derivatization reagent used for selective silylation of hydroxyl groups and carboxylic acid without affecting the amino group. It employed as an intermediate for the synthesis of imidazole derivatives. It plays an essential role to synthesize polysubstituted chiral spirotetrahydropyrans. It is also used in the quantification of pintol after derivatization by gas chromatography/ mass spectrometry.
Solubility
Miscible with most common organic solvents. It decomposes in water.
Notes
Moisture and light sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong acids.
1-(Trimethylsilyl)imidazole acts as a silylating agent for alcohols, carbohydrates and 1,3-dicarbonyl compounds. It is a derivatization reagent used for selective silylation of hydroxyl groups and carboxylic acid without affecting the amino group. It employed as an intermediate for the synthesis of imidazole derivatives. It plays an essential role to synthesize polysubstituted chiral spirotetrahydropyrans. It is also used in the quantification of pintol after derivatization by gas chromatography/ mass spectrometry.
Solubility
Miscible with most common organic solvents. It decomposes in water.
Notes
Moisture and light sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Powerful silylating agent (see Appendix 4) for a wide range of functional groups. O-Silylation in the presence of TBAF (0.02 equiv.) occurs under very mild conditions: Tetrahedron Lett., 35, 8409 (1994).
- Acanski, M. M.; Vujic, D. N. Comparing sugar components of cereal and pseudocereal flour by GC-MS analysis. Food Chem. 2014, 145, 743-748.
- Bu, J.; Rhee, H.-K. Silylation of Ti-MCM-41 by trimethylsilyl-imidazole and its effect on the olefin epoxidation with aqueous H2O2. Catal. Lett. 2000, 66 (4), 245-249.
- Jacolot, M.; Jean, M.; Levoin, N.; Weghe, P. The Prins Reaction Using Ketones: Rationalization and Application toward the Synthesis of the Portentol Skeleton. Org. Lett. 2012, 14 (1), 58-61.