Search Thermo Fisher Scientific
Thermo Scientific Chemicals
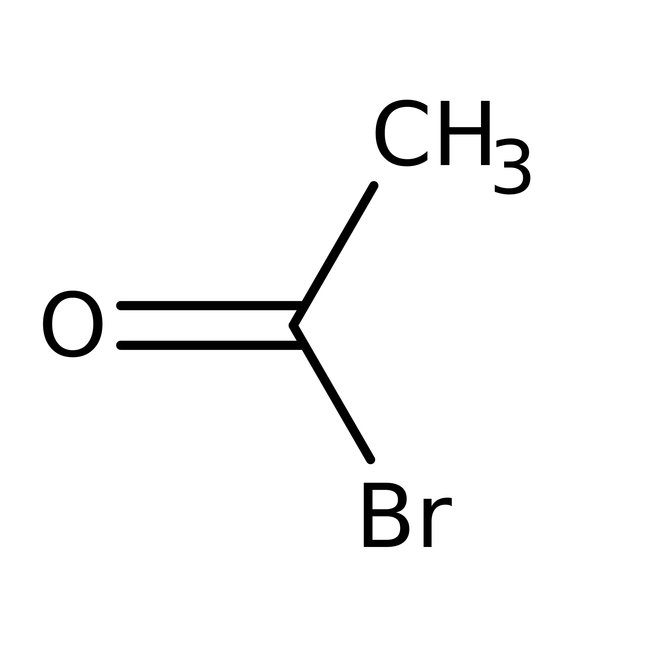
Acetyl bromide, 98%
CAS: 506-96-7 | C2H3BrO | 122.949 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12485.22 | 100 g |
Catalog number ALFA12485.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialAcetyl Bromide
CAS506-96-7
Health Hazard 1H227-H314
Health Hazard 2GHS H Statement
H314-H318-H227
H314-H318-H227
Health Hazard 3P210-P260-P264b-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P370+P378q-P501c
View more
Acetyl Bromide is used as an acetylating agent in the synthesis of fine chemicals, agrochemicals and pharmaceuticals. It is also used as an intermediate for dyes. Further, it reacts with alcohols and amines to produce acetate esters and acetamides. In addition to this, it is involved in the synthetic process of the anti-HIV agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Acetyl Bromide is used as an acetylating agent in the synthesis of fine chemicals, agrochemicals and pharmaceuticals. It is also used as an intermediate for dyes. Further, it reacts with alcohols and amines to produce acetate esters and acetamides. In addition to this, it is involved in the synthetic process of the anti-HIV agent.
Solubility
Miscible with ether, chloroform, benzene and acetone.
Notes
Incompatible with strong oxidizing agents, strong bases and alcohols
Acetyl Bromide is used as an acetylating agent in the synthesis of fine chemicals, agrochemicals and pharmaceuticals. It is also used as an intermediate for dyes. Further, it reacts with alcohols and amines to produce acetate esters and acetamides. In addition to this, it is involved in the synthetic process of the anti-HIV agent.
Solubility
Miscible with ether, chloroform, benzene and acetone.
Notes
Incompatible with strong oxidizing agents, strong bases and alcohols
RUO – Research Use Only
General References:
- In the presence of ZnCl2, adds to a wide range of aldehydes, giving ɑ-bromoalkyl acetates: Helv. Chim Acta, 61, 2047, 2059, 2165 (1978); Synthesis, 593 (1978).
- In combination with TFA, OH groups can be acetylated selectively in the presence of primary and secondary amines: J. Med. Chem., 16, 630 (1973); 17, 427 (1974); Synthesis, 249 (1975).
- Fukushima, R. S.; Kerley, M. S.; Ramos, M. H.; Porter, J. H.; Kallenbach, R. L. Comparison of acetyl bromide lignin with acid detergent lignin and Klason lignin and correlation with in vitro forage degradability. Anim. Feed Sci. Technol. 2015, 201, 25-37.
- Grabber, J. H.; Santoro, N.; Foster, C. E.; Elumalai, S.; Ralph, J.; Pan, X. Incorporation of Flavonoid Derivatives or Pentagalloyl Glucose into Lignin Enhances Cell Wall Saccharification Following Mild Alkaline or Acidic Pretreatments. BioEnerg. Res. 2015, 8 (3), 1391-1400.