Search Thermo Fisher Scientific
Thermo Scientific Chemicals
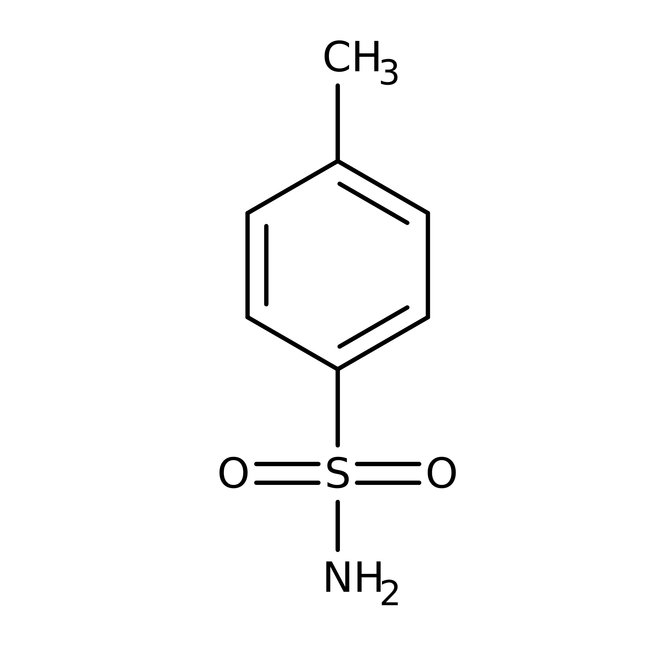
p-Toluenesulfonamide, 98+%
CAS: 70-55-3 | C7H9NO2S | 171.214 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12156.30 | 250 g |
Catalog number ALFA12156.30
Price (MYR)
310.00
EA
Quantity:
250 g
Price (MYR)
310.00
EA
Specifications
Chemical Name or Materialp-Toluenesulfonamide
CAS70-55-3
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
p-Toluene sulfonamide was used to prepare the precursor required for synthesis of ethyl 6-phenyl-1-tosyl-1,2,5,6-tetrahydropyridine-3-carboxylate. It act as a derivative of ammonia activated to alkylation by alkyl halides is exemplified by the synthesis of N-tosyl-2,3-dihydroisoindole from o-xylylene dibromide. It is a precursor of N-tosyl imines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
p-Toluene sulfonamide was used to prepare the precursor required for synthesis of ethyl 6-phenyl-1-tosyl-1,2,5,6-tetrahydropyridine-3-carboxylate. It act as a derivative of ammonia activated to alkylation by alkyl halides is exemplified by the synthesis of N-tosyl-2,3-dihydroisoindole from o-xylylene dibromide. It is a precursor of N-tosyl imines.
Solubility
Soluble in water.
Notes
Store in cool, dry place, in well sealed container. Store away from oxidizing agents.
p-Toluene sulfonamide was used to prepare the precursor required for synthesis of ethyl 6-phenyl-1-tosyl-1,2,5,6-tetrahydropyridine-3-carboxylate. It act as a derivative of ammonia activated to alkylation by alkyl halides is exemplified by the synthesis of N-tosyl-2,3-dihydroisoindole from o-xylylene dibromide. It is a precursor of N-tosyl imines.
Solubility
Soluble in water.
Notes
Store in cool, dry place, in well sealed container. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Kui Lu; Ohyun Kwon. Phosphine-Catalyzed [4+2] Annulation: Synthesis of Ethyl 6-Phenyl-1-Tosyl-1,2,5,6-Tetrahydropyridine-3-Carboxylate. Organic Syntheses. 2009, 2009 (86), 212-224.
- D. S. Tarbell; Clay Weaver. The Condensation of Sulfoxides with p-Toluenesulfonamide and Substituted Acetamides. J. Am. Chem. Soc. 1941, 63 (11), 2939-2942.
- Use of p-toluenesulfonamide as a derivative of ammonia activated to alkylation by alkyl halides is exemplified by the synthesis of N-tosyl-2,3-dihydroisoindole from o-xylylene dibromide: Org. Synth. Coll., 5, 1064 (1973). Similarly, has been used as the precursor of nitrogen-containing crown ethers; see, e.g.: J. Org. Chem., 53, 5292 (1988).
- Precursor of N-tosyl imines, which are useful intermediates in synthesis: Synlett, 2097 (2003).