Search Thermo Fisher Scientific
Thermo Scientific Chemicals
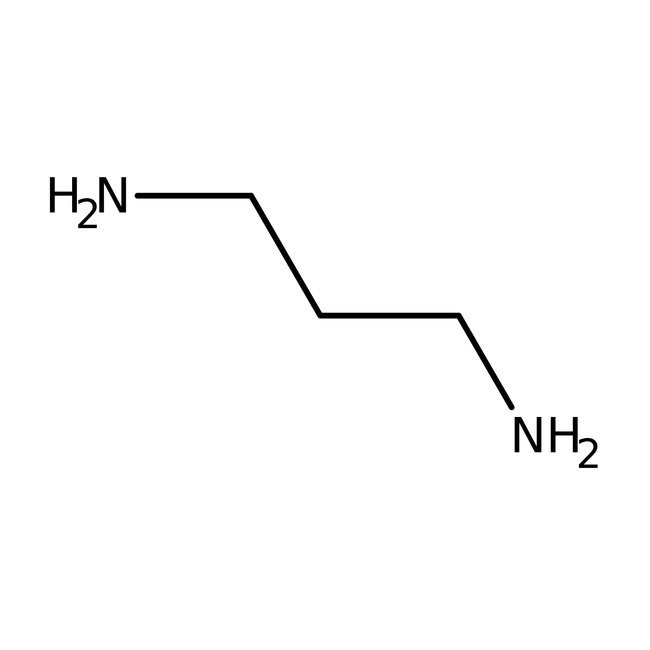
1,3-Diaminopropane, 98%
CAS: 109-76-2 | C3H10N2 | 74.127 g/mol
Catalog number ALFA11932.AP
Price (MYR)
573.00
EA
Quantity:
500 mL
Price (MYR)
573.00
EA
Specifications
Chemical Name or Material1,3-Diaminopropane
CAS109-76-2
Health Hazard 1H226-H302-H310-H314-H317-H334-H335
Health Hazard 2GHS H Statement
H310-H314-H318-H226-H302
Fatal in contact with skin.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Flammable liquid and vapor.
Harmful if swallowed.
H310-H314-H318-H226-H302
Fatal in contact with skin.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Flammable liquid and vapor.
Harmful if swallowed.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P262-P264b-P270-P271-P272-P280-P285-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P333+P313-P363-P370+P378q-P501c
View more
1,3-Diaminopropane is commonly used in the preparation of cholinesterase inhibitors and thrombosis inhibitors. It is also used as a polymer cross linkage agent, polyurethane extender and spray coatings. It is involved in the preparation of agrochemicals and pharmaceutical intermediates. Further, it acts as a building block in the synthesis of heterocyclic compounds, which finds application in textile finishing. In addition to this, it is used as a ligand and form coordination complexes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Diaminopropane is commonly used in the preparation of cholinesterase inhibitors and thrombosis inhibitors. It is also used as a polymer cross linkage agent, polyurethane extender and spray coatings. It is involved in the preparation of agrochemicals and pharmaceutical intermediates. Further, it acts as a building block in the synthesis of heterocyclic compounds, which finds application in textile finishing. In addition to this, it is used as a ligand and form coordination complexes.
Solubility
Miscible with water.
Notes
Hygroscopic. Air sensitive. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents and carbon dioxide.
1,3-Diaminopropane is commonly used in the preparation of cholinesterase inhibitors and thrombosis inhibitors. It is also used as a polymer cross linkage agent, polyurethane extender and spray coatings. It is involved in the preparation of agrochemicals and pharmaceutical intermediates. Further, it acts as a building block in the synthesis of heterocyclic compounds, which finds application in textile finishing. In addition to this, it is used as a ligand and form coordination complexes.
Solubility
Miscible with water.
Notes
Hygroscopic. Air sensitive. Incompatible with acids, acid chlorides, acid anhydrides, strong oxidizing agents and carbon dioxide.
RUO – Research Use Only
General References:
- The potassium derivative (KAPA) is a very strong base, valuable for its ability to cause the migration of acetylenic unsaturation to the terminal positions of alkynes (known as the zip reaction). In the presence of excess amine, reaction occurs within seconds; the driving force is the formation of a stable acetylide anion: J. Am. Chem. Soc., 97, 891 (1975). For example, with acetylenic carbinols: J. Chem. Soc., Chem. Commun., 959 (1976); Tetrahedron Lett., 2565 (1976); Can. J. Chem., 62, 1333 (1984). For a further example (2- to 9-decynol), using a combination of Potassium tert-butoxide, A13947, and Li 3-aminopropanamide, see: Org. Synth. Coll., 8, 146 (1993). This technique avoids the use of KH. For the use of KAPA for the isomerization of ß-pinene to the thermodynamically-more stable ɑ-pinene, see: Org. Synth. Coll., 8, 553 (1993).
- For procedures for the generation of Na or K 3-amino-propylamides from the metals by ultrasound irradiation or in the presence of Fe(NO3)3, see: J. Org. Chem., 49, 2494 (1984).
- Bag, P.; Ghosh, A. B.; Dutta, A. K.; Flörke, U.; Nag, K. Sandwich-type lanthanide(III) complexes of a tetraiminodiphenol macrocyclic ligand derived from 4-methyl-2,6-diformylphenol and 1,3-diaminopropane: structure, NMR and luminescence. Polyhedron 2015, 102, 539-548.
- Liang, K. L.; Lin, Y. F.; Leron, R. B.; Li, M. H. Molar heat capacity of aqueous solutions of 1, 3-diaminopropane and 1, 4-diaminobutane and their piperazine blends. Thermochim. Acta 2015, 616, 42-48.