Search Thermo Fisher Scientific
Thermo Scientific Chemicals
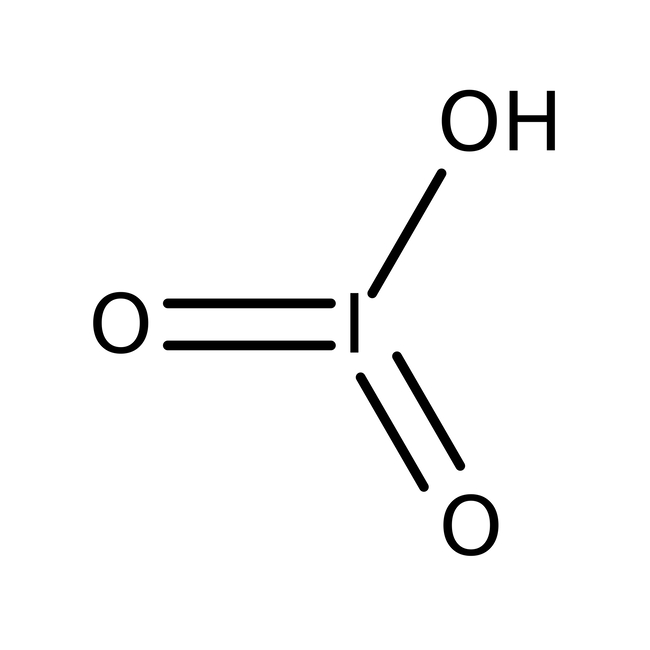
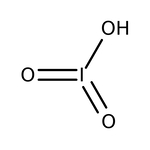
Iodic acid, 99%
CAS: 7782-68-5 | ILiO3 | 181.84 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11925.18 | 50 g |
Catalog number ALFA11925.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or MaterialIodic acid
CAS7782-68-5
Melting Point110°C (decomposition)
Health Hazard 1H272-H314-H335
Density4.629
View more
Iodic acid is used in analytical chemistry laboratories to standardize solutions of both weak and strong bases using methyl red or methyl orange as the indicator. It acts as a key starting material to synthesize sodium or potassium iodate, thereby increasing the iodine content of salt.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Iodic acid is used in analytical chemistry laboratories to standardize solutions of both weak and strong bases using methyl red or methyl orange as the indicator. It acts as a key starting material to synthesize sodium or potassium iodate, thereby increasing the iodine content of salt.
Solubility
Soluble in water and methanol.
Notes
Light sensitive. Incompatible with reducing agents, alcohols and organic materials.
Iodic acid is used in analytical chemistry laboratories to standardize solutions of both weak and strong bases using methyl red or methyl orange as the indicator. It acts as a key starting material to synthesize sodium or potassium iodate, thereby increasing the iodine content of salt.
Solubility
Soluble in water and methanol.
Notes
Light sensitive. Incompatible with reducing agents, alcohols and organic materials.
RUO – Research Use Only
General References:
- Oxidizing agent. For selective oxidation of thiols to disulfides and sulfides to sulfoxides by aqueous iodic acid, see: Russ. J. Org. Chem., 37, 1340 (2001). Carbonyl compounds can be regenerated from their oximes in DCM: Tetrahedron Lett., 43, 4023 (2002). For a brief survey of uses in organic synthesis, see: Synlett, 2347 (2006).
- Murray, B. J.; Haddrell, A. E.; Peppe, S.; Davies, J. F.; Reid, J. P.; O'sullivan, D.; Price, H. C.; Kumar, R.; Saunders, R. W.; Plane, J. M. C.; Umo, N. S.; Wilson, T. W. Glass formation and unusual hygroscopic growth of iodic acid solution droplets with relevance for iodine mediated particle formation in the marine boundary layer. Atmos. Chem. Phys. 2012, 12 (18), 8575-8587.
- Arjunan, S.; Bhaskaran, A.; Kumar, R. M.; Mohan, R.; Jayavel, R. Effect of iodic acid dopant on the growth and structural, optical, and electrical properties of L-arginine phosphate single crystals. Mater. Manuf. Processes 2012, 27 (1), 49-52.