Search Thermo Fisher Scientific
Thermo Scientific Chemicals
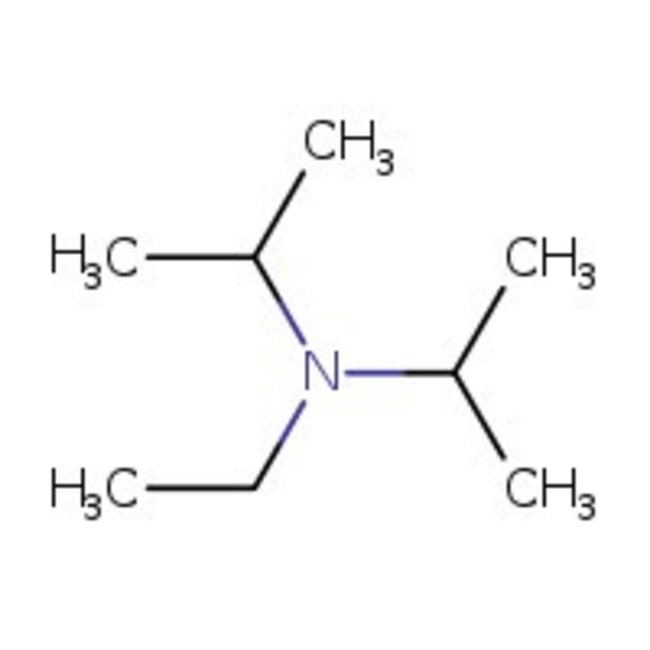
N-Ethyldiisopropylamine, 99%
CAS: 7087-68-5 | C8H19N | 129.247 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11801.AP | 500 mL |
Catalog number ALFA11801.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or MaterialN-Ethyldiisopropylamine
CAS7087-68-5
Health Hazard 1H225-H290-H302-H318-H331-H335
Health Hazard 3P210-P233-P234-P235-P240-P241-P242-P243-P261-P264b-P270-P271-P280-P301+P312-P303+P361+P353-P304+P340-P305+P351+P338-P310-P311-P330-P370+P378q-P390-P501c
Melting Point−46°C
View more
N-Ethyldiisopropylamine is used to make or formulate industrial products. It is also used in organic chemistry as a base. It acts as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. It reacts with monoactivated Michael acceptors to afford symmetrical sulfones. Further, it is used to prepare heterocyclic compound, sscorpionine by a reaction with disulfur dichloride using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a catalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Ethyldiisopropylamine is used to make or formulate industrial products. It is also used in organic chemistry as a base. It acts as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. It reacts with monoactivated Michael acceptors to afford symmetrical sulfones. Further, it is used to prepare heterocyclic compound, sscorpionine by a reaction with disulfur dichloride using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a catalyst.
Solubility
Miscible with water and ethanol.
Notes
Incompatible with acids, acid chlorides, acid anhydrides, carbon dioxide, copper, brass, rubber, oxidizing agents, nitrates, peroxides, aldehydes and metals.
N-Ethyldiisopropylamine is used to make or formulate industrial products. It is also used in organic chemistry as a base. It acts as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. It reacts with monoactivated Michael acceptors to afford symmetrical sulfones. Further, it is used to prepare heterocyclic compound, sscorpionine by a reaction with disulfur dichloride using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a catalyst.
Solubility
Miscible with water and ethanol.
Notes
Incompatible with acids, acid chlorides, acid anhydrides, carbon dioxide, copper, brass, rubber, oxidizing agents, nitrates, peroxides, aldehydes and metals.
RUO – Research Use Only
General References:
- Hindered non-nucleophilic base with high proton affinity: Chem. Ber., 91, 380 (1958).
- For use in the introduction of TBDMS protecting groups, see tert-Butyl dimethyl chlorosilane, A13064 . Used in preparation of silyl enol ethers from both aldehydes and ketones: Helv. Chim. Acta, 60, 181 (1977).
- Useful base in peptide coupling reactions: J. Am. Chem. Soc., 91, 6488 (1969). See Appendix 6.
- Has been recommended as a base for the Horner-Wadsworth-Emmons olefination reaction, where the reagents contain groups sensitive to base-catalyzed epimerization or condensation. The reaction is carried out in the presence of LiCl, and gives high (E):(Z) ratios: Tetrahedron Lett., 25, 2183 (1984).
- Vembu, S.; Pazhamalai, S.; Gopalakrishnan, M. Potential antibacterial activity of triazine dendrimer: Synthesis and controllable drug release properties. Bioorg. Med. Chem. 2015, 23 (15), 4561-4566.
- Manandhar, Y.; Bahadur, K. C. T.; Wang, W.; Uzawa, T.; Aigaki, T.; Ito, Y. In vitro selection of a peptide aptamer that changes fluorescence in response to verotoxin. Biotechnol. Lett 2015, 37 (3), 619-625.