Search Thermo Fisher Scientific
Thermo Scientific Chemicals
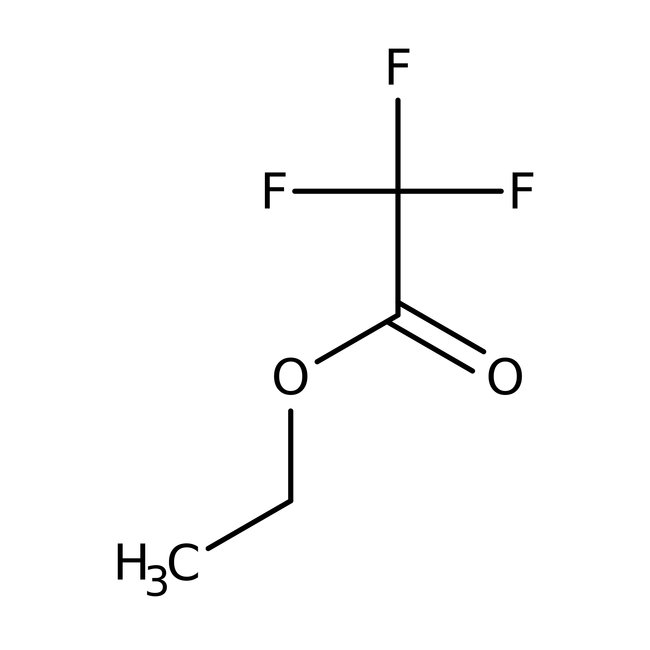
Ethyl trifluoroacetate, 99%
CAS: 383-63-1 | C4H5F3O2 | 142.077 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11520.18 | 50 g |
Catalog number ALFA11520.18
Price (MYR)
440.00
EA
Quantity:
50 g
Price (MYR)
440.00
EA
Specifications
Chemical Name or MaterialEthyl trifluoroacetate
CAS383-63-1
Health Hazard 1H225-H319
Health Hazard 2GHS H Statement
H225-H314-H318
Highly flammable liquid and vapor.
Causes severe skin burns and eye damage.
Causes serious eye damage.
H225-H314-H318
Highly flammable liquid and vapor.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P210-P233-P240-P241-P242-P243-P264b-P280-P303+P361+P353-P305+P351+P338-P370+P378q-P501c
View more
Ethyl trifluoroacetate is used as an intermediate in organic synthesis to prepare organic fluorine compounds like 3-ethyl-1-methylimidazolium trifluoroacetate (EMITA). It is involved in the syntheses of various pharmaceutically active molecules and agricultural products. It is also useful for the preparation of tri fluoroacylated compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl trifluoroacetate is used as an intermediate in organic synthesis to prepare organic fluorine compounds like 3-ethyl-1-methylimidazolium trifluoroacetate (EMITA). It is involved in the syntheses of various pharmaceutically active molecules and agricultural products. It is also useful for the preparation of tri fluoroacylated compounds.
Solubility
Slightly miscible with water. Miscible with chloroform and methanol.
Notes
Moisture Sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong bases and strong acids.
Ethyl trifluoroacetate is used as an intermediate in organic synthesis to prepare organic fluorine compounds like 3-ethyl-1-methylimidazolium trifluoroacetate (EMITA). It is involved in the syntheses of various pharmaceutically active molecules and agricultural products. It is also useful for the preparation of tri fluoroacylated compounds.
Solubility
Slightly miscible with water. Miscible with chloroform and methanol.
Notes
Moisture Sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong bases and strong acids.
RUO – Research Use Only
General References:
- Reagent for selective trifluoroacetylation of amines. Primary amines can be protected in the presence of secondary, and diamines selectively monoprotected. Primary amines can also be differentiated in terms of steric hindrance: Tetrahedron Lett., 36, 7357 (1995).
- Esters of perfluoroalkyl carboxylic acids undergo Wittig reactions, for example with the ylide from (3-Phenyl propyl) triphenyl phosphonium bromide, A12669, leading to 1-perfluoroalkyl enol ethers, which are useful precursors of various fluorinated derivatives: J. Org. Chem., 57, 3807 (1992); Org. Synth., 75, 153 (1997):
- Bhattacharjee, D.; Tiwari, L.; Singh, H. J.; Mishra, B. K.; Deka, R. C. Theoretical investigation on mechanism, kinetics and thermochemistry of gas-phase reactions of ethyl trifluoroacetate with OH radicals. J. Fluorine Chem. 2015, 178, 79-85.
- Huang, Z.; Jiang, H.; Li, L.; Wang, H.; Qiu, T. Density, viscosity, and saturated vapor pressure of ethyl trifluoroacetate. J. Chem. Thermodyn. 2015, 86, 75-79.