Search Thermo Fisher Scientific
Thermo Scientific Chemicals
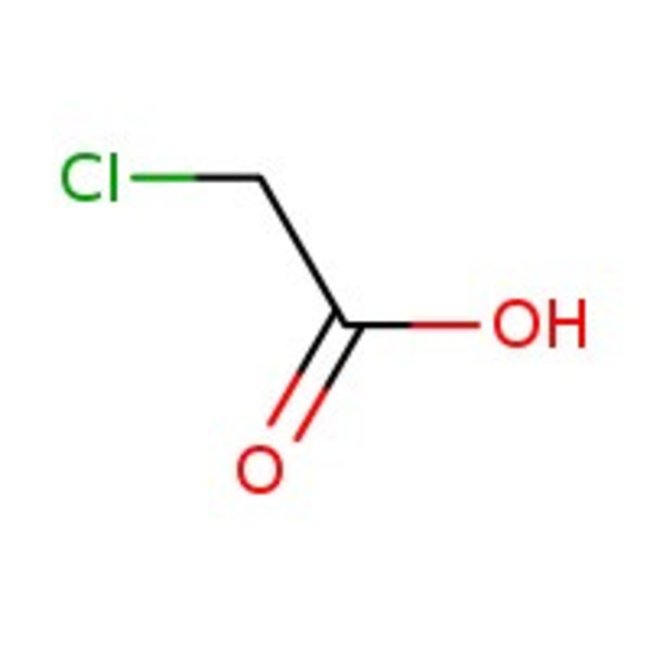
Chloroacetic acid, 99%
CAS: 79-11-8 | C2H3ClO2 | 94.494 g/mol
Catalog number ALFA11482.30
Price (MYR)
259.00
EA
Quantity:
250 g
Price (MYR)
259.00
EA
Specifications
Chemical Name or MaterialChloroacetic acid
CAS79-11-8
Health Hazard 1H301+H311-H314-H330-H335
Health Hazard 2GHS H Statement
H301-H311-H331-H314
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes severe skin burns and eye damage.
H301-H311-H331-H314
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Health Hazard 3P260-P264b-P270-P271-P280-P284-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
View more
Chloroacetic acid is used in the production of pharmaceuticals, dyes, detergents, synthetic caffeine, glycine and thioglycolic acid, which acts as a stabilizer in PVC. It acts as a precursor to glyphosate and adrenaline. It also serves as a preservative and bacteriostat. Further, it is used as surface active agents and photosensitive chemicals. It is also used in the O-alkylation of salicylaldehyde and an active component in cosmetics. In addition, it is involved in the preparation of thickening agent carboxymethyl cellulose and carboxymethyl starch.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Chloroacetic acid is used in the production of pharmaceuticals, dyes, detergents, synthetic caffeine, glycine and thioglycolic acid, which acts as a stabilizer in PVC. It acts as a precursor to glyphosate and adrenaline. It also serves as a preservative and bacteriostat. Further, it is used as surface active agents and photosensitive chemicals. It is also used in the O-alkylation of salicylaldehyde and an active component in cosmetics. In addition, it is involved in the preparation of thickening agent carboxymethyl cellulose and carboxymethyl starch.
Solubility
Soluble in water, methanol, acetone, diethyl ether, benzene, chloroform and ethanol.
Notes
Incompatible with strong oxidizing agents, strong bases and strong reducing agents.
Chloroacetic acid is used in the production of pharmaceuticals, dyes, detergents, synthetic caffeine, glycine and thioglycolic acid, which acts as a stabilizer in PVC. It acts as a precursor to glyphosate and adrenaline. It also serves as a preservative and bacteriostat. Further, it is used as surface active agents and photosensitive chemicals. It is also used in the O-alkylation of salicylaldehyde and an active component in cosmetics. In addition, it is involved in the preparation of thickening agent carboxymethyl cellulose and carboxymethyl starch.
Solubility
Soluble in water, methanol, acetone, diethyl ether, benzene, chloroform and ethanol.
Notes
Incompatible with strong oxidizing agents, strong bases and strong reducing agents.
RUO – Research Use Only
General References:
- A convenient one-pot alternative to the Knoevenagel or Horner-Wadsworth-Emmons reactions for the synthesis of ɑ-unsubstituted cinnamic acids involves the reaction of chloroacetic acid with dimethyl phosphite in the presence of sodium methoxide, and treatment of the resulting phosphonoacetate salt with a benzaldehyde: J. Org. Chem., 46, 2514 (1981):
- For a more general version of this reaction, applicable to the synthesis of a variety of substituted acrylic acids, see 2-Bromopropionic acid, A17569 .
- Scialdone, O.; Galia, A.; Sabatino, S.; Mira, D.; Amatore, C. Electrochemical Conversion of Dichloroacetic Acid to Chloroacetic Acid in a Microfluidic Stack and in a Series of Microfluidic Reactors. Chem. Electro. Chem. 2015, 2 (5), 684-690.
- Teng, Y.; Liu, Y.; Jiang, J. Preparation of Carboxyl-End-Group Poly(methyl methacrylate) and Polystyrene with Low Polydispersity by Atom Transfer Radical Polymerization Initiated with Chloroacetic Acid. Asian J. Chem. 2015, 27 (9), 3254-3258.