Search Thermo Fisher Scientific
Thermo Scientific Chemicals
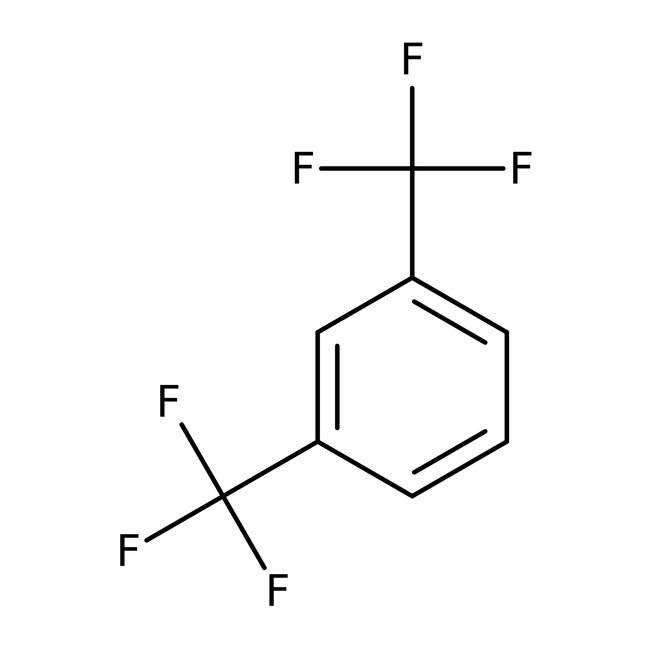
1,3-Bis(trifluoromethyl)benzene, 98+%
CAS: 402-31-3 | C8H4F6 | 214.11 g/mol
Catalog number ALFA11360.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material1,3-Bis (trifluoromethyl)benzene
CAS402-31-3
Health Hazard 1H226-H315-H319-H335
Health Hazard 2GHS H Statement
H226-H315-H319-H335
Flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H226-H315-H319-H335
Flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P312-P332+P313-P363-P370+P378q-P501c
View more
1,3-Bis(trifluoromethyl)benzene undergoes regioselective metalation and subsequent carboxylation at position 2 to give 2,6-bis(trifluoromethyl)benzoic acid. a convenient, selective synthesis of bis[2,4-bis(trifluoromethyl)phenyl]phosphane derivatives with 1,3-bis(trifluoromethyl)benzene as starting material.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Bis(trifluoromethyl)benzene undergoes regioselective metalation and subsequent carboxylation at position 2 to give 2,6-bis(trifluoromethyl)benzoic acid. a convenient, selective synthesis of bis[2,4-bis(trifluoromethyl)phenyl]phosphane derivatives with 1,3-bis(trifluoromethyl)benzene as starting material.
Solubility
Insoluble in water. Soluble in alcohol, ether, benzene.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
1,3-Bis(trifluoromethyl)benzene undergoes regioselective metalation and subsequent carboxylation at position 2 to give 2,6-bis(trifluoromethyl)benzoic acid. a convenient, selective synthesis of bis[2,4-bis(trifluoromethyl)phenyl]phosphane derivatives with 1,3-bis(trifluoromethyl)benzene as starting material.
Solubility
Insoluble in water. Soluble in alcohol, ether, benzene.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
General References:
- Aeberli P,; Houlihan WJ. The Metalation of 1, 3-Bis (trifluoromethyl) benzene by n butyllithium.. J. Organomet. Chem. 1974, 64 (3), 321-25.
- Berthold Hoge Dr.,; Boris Kurscheid,; Sebastian Peuker,; Wieland Tyrra,; Hendrik T. M. Fischer. A Convenient, Selective Synthesis of Bis[2,4-bis(trifluoromethyl)phenyl]phosphane Derivatives Starting from 1,3-Bis(trifluoromethyl)benzene† . Zeitschrift für anorganische und allgemeine Chemie. 2007, 633 (10) ,679-1685 pg no.
- Metallation with n-BuLi in diethyl ether is unselective, giving, on quenching with CO2, a mixture of 2,4- and 2,6-bis(trifluoromethyl)benzoic acids: J. Organomet. Chem., 67, 321 (1974). The superbasic n-BuLi/KO-t-Bu combination leads to almost exclusive 2-metallation, allowing the 2,6-bis(trifluoromethyl) acid to be isolated in 78% yield. In contrast, a combination of sec-BuLi and N,N,N',N',N'-pentamethyldiethylenetriamine leads to the 2,4-bis(trifluoromethyl) acid as the main product: Synlett, 747 (1990).