Search Thermo Fisher Scientific
Thermo Scientific Chemicals
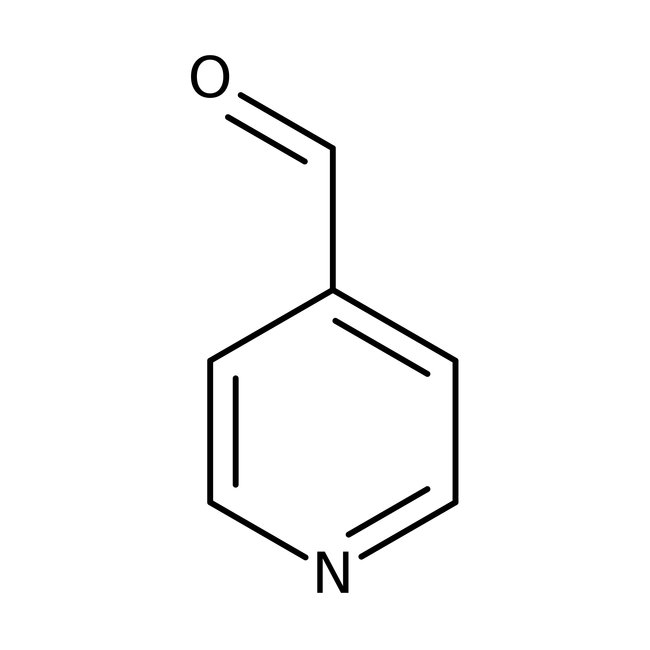
Pyridine-4-carboxaldehyde, 97%
CAS: 872-85-5 | C6H5NO | 107.112 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10656.14 | 25 g |
Catalog number ALFA10656.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialPyridine-4-carboxaldehyde
CAS872-85-5
Health Hazard 1H227-H314-H317-H332
Health Hazard 2GHS H Statement
H315-H319-H317-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause an allergic skin reaction.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H317-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause an allergic skin reaction.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P260-P264b-P271-P272-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P333+P313-P363-P370+P378q-P501c
View more
Pyridine-4-carboxaldehyde is an heterocyclic building block used for the synthesis of various pharmaceutical compounds, such as new 1,4-dihydropyridin-4-yl-phenoxyacetohydrazones, having anticonvulsant and anti-inflammatory properties.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Pyridine-4-carboxaldehyde is an heterocyclic building block used for the synthesis of various pharmaceutical compounds, such as new 1,4-dihydropyridin-4-yl-phenoxyacetohydrazones, having anticonvulsant and anti-inflammatory properties.
Solubility
Soluble in water (20 mg/ml at 20°C), ethanol and acetone.
Notes
It is air and light sensitive. Incompatible with oxidizing agents.
Pyridine-4-carboxaldehyde is an heterocyclic building block used for the synthesis of various pharmaceutical compounds, such as new 1,4-dihydropyridin-4-yl-phenoxyacetohydrazones, having anticonvulsant and anti-inflammatory properties.
Solubility
Soluble in water (20 mg/ml at 20°C), ethanol and acetone.
Notes
It is air and light sensitive. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Nand K.Singh; Rajesh Tripathi. Synthesis and structural characterization of 3d-metal complexes of pyridine-4-carboxaldehyde thionicotinoyl hydrazone. Transition Metal Chemistry. 1988, 13, (5),346-350
- A.Martin; B.Lucke; H.J.Niclas; A.Forster. Vapor-phase oxidation of 4-picoline to pyridine-4-carboxaldehyde on a vanadium phosphate catalysts. Reaction Kinetics and Catalysis Letters. 1991, 43, (2),583-588
- In the presence of DBU, converts amines to carbonyl compounds, e.g. benzylamines to benzaldehydes: Synthesis, 756 (1982):
- The reaction is also useful for the conversion of ɑ-amino acids to ɑ-keto acids.