Search Thermo Fisher Scientific
Thermo Scientific Chemicals
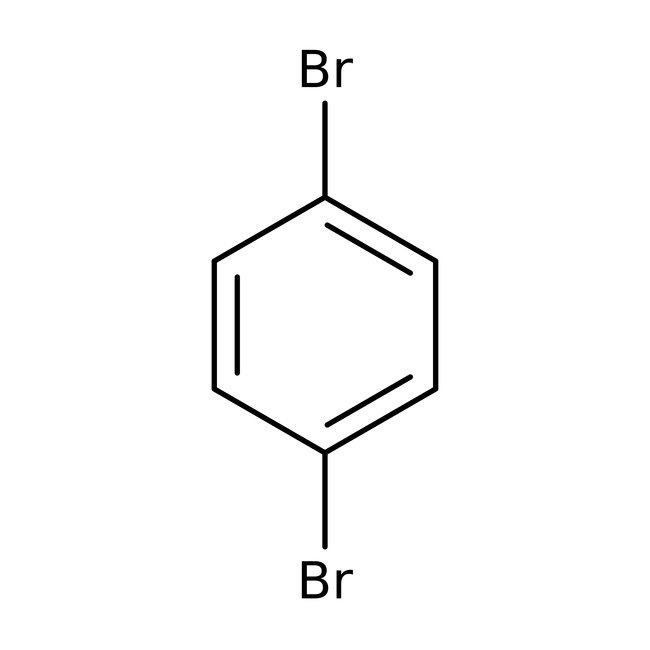
1,4-Dibromobenzene, 98%
CAS: 106-37-6 | C6H4Br2 | 235.91 g/mol
Catalog number ALFA10517.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material1,4-Dibromobenzene
CAS106-37-6
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
1,4-Dibromobenzene is used in the synthesis of 2,5-dibromoacetophenone, triarylamines and trans- stilbenes. It is widely used as a heavy liquid solvent as well as a motor oil additive. It finds application as an intermediate in the manufacture of organic chemicals such as dyes, pharmaceuticals and flame retardants for polymeric materials.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4-Dibromobenzene is used in the synthesis of 2,5-dibromoacetophenone, triarylamines and trans- stilbenes. It is widely used as a heavy liquid solvent as well as a motor oil additive. It finds application as an intermediate in the manufacture of organic chemicals such as dyes, pharmaceuticals and flame retardants for polymeric materials.
Solubility
Soluble in alcohol, benzene, chloroform, toluene and carbon tetrachloride. Insoluble in ether.
Notes
Avoid excessive heat and light. Incompatible with strong oxidizing agents.
1,4-Dibromobenzene is used in the synthesis of 2,5-dibromoacetophenone, triarylamines and trans- stilbenes. It is widely used as a heavy liquid solvent as well as a motor oil additive. It finds application as an intermediate in the manufacture of organic chemicals such as dyes, pharmaceuticals and flame retardants for polymeric materials.
Solubility
Soluble in alcohol, benzene, chloroform, toluene and carbon tetrachloride. Insoluble in ether.
Notes
Avoid excessive heat and light. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- In the presence of NiCl2, cross-coupling with aryl Grignards gives p-terphenyls: Synthesis, 147 (1990).
- Cano, N. H.; Ballari, M. S.; Lopez, A. G.; Santiago, A. N. New Synthesis and Biological Evaluation of Benzothiazole Derivates as Antifungal Agents. J. Agric. Food Chem. 2015, 63 (14), 3681-3686.
- Vazdar, M.; Mitchell, A. S.; Warrener, R. N.; Margetic, D. High pressure promoted exchange of guests from hemicarceplexes. Tetrahedron 2015, 71 (4), 550-553.