Search Thermo Fisher Scientific
Thermo Scientific Chemicals
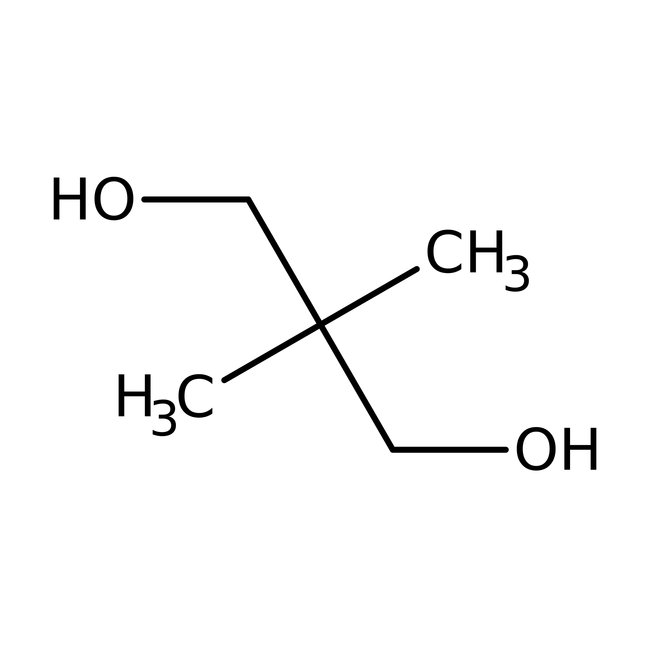
2,2-Dimethyl-1,3-propanediol, 99%
CAS: 126-30-7 | C5H12O2 | 104.15 g/mol
Catalog number ALFA10340.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material2,2-Dimethyl-1,3-propanediol
CAS126-30-7
Health Hazard 1H318-H500
Health Hazard 2GHS H Statement
H318
Causes serious eye damage.
H318
Causes serious eye damage.
Health Hazard 3P280-P305+P351+P338-P310
View more
Used for protection of carbonyl groups as 4,4-dimethyl-1,3-dioxanes, often preferred to the more usual 1,3-dioxolanes due to easier separation and greater stability, for protection of carbonyl group of 4-bromo-2-propanone using p-toluenesulfonic acid in the presence of triethyl orthoformate. It is used in the synthesis of polyesters, paints, lubricants, and plasticizers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Used for protection of carbonyl groups as 4,4-dimethyl-1,3-dioxanes, often preferred to the more usual 1,3-dioxolanes due to easier separation and greater stability, for protection of carbonyl group of 4-bromo-2-propanone using p-toluenesulfonic acid in the presence of triethyl orthoformate. It is used in the synthesis of polyesters, paints, lubricants, and plasticizers.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with oxidizing agents, water/moisture.
Used for protection of carbonyl groups as 4,4-dimethyl-1,3-dioxanes, often preferred to the more usual 1,3-dioxolanes due to easier separation and greater stability, for protection of carbonyl group of 4-bromo-2-propanone using p-toluenesulfonic acid in the presence of triethyl orthoformate. It is used in the synthesis of polyesters, paints, lubricants, and plasticizers.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with oxidizing agents, water/moisture.
RUO – Research Use Only
General References:
- Richard L. Mcconnell; H. W. CooverJr. Phosphorus-Containing Derivatives of 2,2-Dimethyl-1,3-propanediol. J. Org. Chem.1959, 24 (5), 630-635.
- Used for protection of carbonyl groups as 4,4-dimethyl-1,3-dioxanes, often preferred to the more usual 1,3-dioxolanes due to easier separation and greater stability. For an example, see 2,3-Butanedione, A14217. For protection of carbonyl group of 4-bromo-2-propanone using p-toluenesulfonic acid in the presence of triethyl orthoformate, see: Org. Synth. Coll., 7, 59 (1990).