Search Thermo Fisher Scientific
Thermo Scientific Chemicals
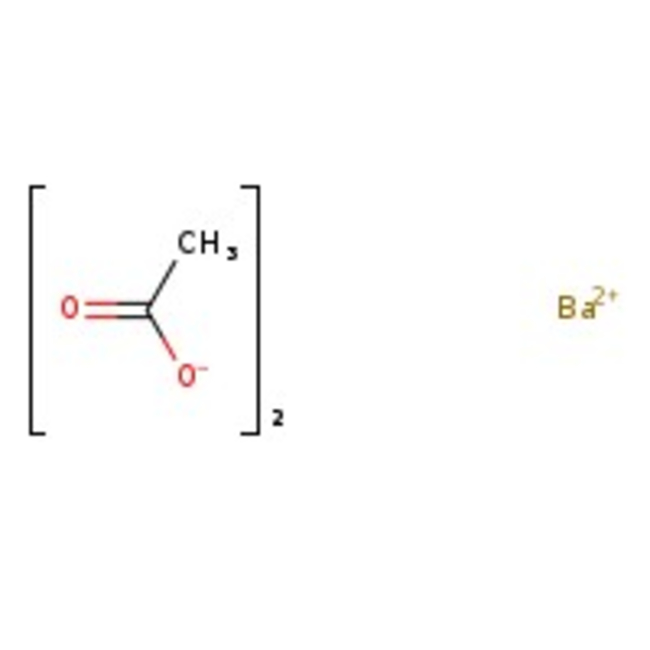
Barium acetate, 99%
CAS: 543-80-6 | C4H6BaO4 | 255.42 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10316.30 | 250 g |
Catalog number ALFA10316.30
Price (MYR)
309.00
EA
Quantity:
250 g
Price (MYR)
309.00
EA
Specifications
Chemical Name or MaterialBarium acetate
CAS543-80-6
Health Hazard 1H302+H332
Health Hazard 2GHS H Statement
H302-H332
Harmful if swallowed.
Harmful if inhaled.
H302-H332
Harmful if swallowed.
Harmful if inhaled.
Health Hazard 3P261-P264b-P270-P271-P301+P312-P304+P340-P312-P330-P501c
View more
It has acted as cationization agent for sensitive multiple reaction monitoring of plasma free fatty acid profiling in a fish oil human intervention. Barium acetate has been used in the process of titrating sulfates of quinine and quinidine in nonaqueous conditions. Electrophoresis in the presence of barium acetate has analytically and preparatively separate glycosaminoglycans. It has been documented to induce severe cardiac disrhythmia from ingestion. It also finds its application as a chemical reagent, textile mordant, catalyst for organic reactions, and paint and varnish driers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It has acted as cationization agent for sensitive multiple reaction monitoring of plasma free fatty acid profiling in a fish oil human intervention. Barium acetate has been used in the process of titrating sulfates of quinine and quinidine in nonaqueous conditions. Electrophoresis in the presence of barium acetate has analytically and preparatively separate glycosaminoglycans. It has been documented to induce severe cardiac disrhythmia from ingestion. It also finds its application as a chemical reagent, textile mordant, catalyst for organic reactions, and paint and varnish driers.
Solubility
Soluble in water at 20°C 588g/L. Insoluble in alcohol.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It has acted as cationization agent for sensitive multiple reaction monitoring of plasma free fatty acid profiling in a fish oil human intervention. Barium acetate has been used in the process of titrating sulfates of quinine and quinidine in nonaqueous conditions. Electrophoresis in the presence of barium acetate has analytically and preparatively separate glycosaminoglycans. It has been documented to induce severe cardiac disrhythmia from ingestion. It also finds its application as a chemical reagent, textile mordant, catalyst for organic reactions, and paint and varnish driers.
Solubility
Soluble in water at 20°C 588g/L. Insoluble in alcohol.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Erland Wessler. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate.Anal. Biochem.,1968,26(3), 439-444.
- Phule, P.P. ; Risbud, S.H. Sol-gel synthesis of barium titanate powders using barium acetate and titanium(IV) isopropoxide.Advanced Ceramic Materials.,1988,3(2).