Search Thermo Fisher Scientific
Thermo Scientific Chemicals
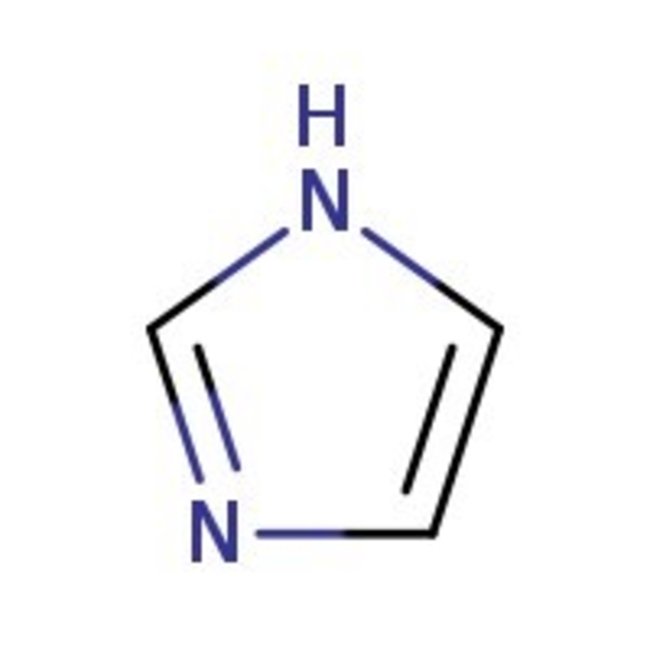
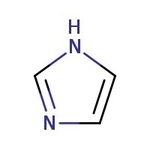
Imidazole, 99%
CAS: 288-32-4 | C3H4N2 | 68.08 g/mol
Catalog number ALFA10221.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialImidazole
CAS288-32-4
Health Hazard 1H302-H314-H335-H360D-H500
Health Hazard 3P201-P202-P260-P264b-P270-P271-P281-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
Melting Point88°C to 92°C
View more
Imidazole is used as a buffer in the range of pH 6.2-7.8. It is also an histamine antagonist. It acts as a chelator and forms complexes with various divalent cations. It is used as a corrosion inhibitor on certain transition metals such as copper. Its derivatives, like polybenzimidazole (PBI), act as fire retardants. It finds application in photography and electronics. Imidazole salts are used as ionic liquids and precursors to stable carbenes. Imidazole derivatives like ketoconazole, miconazole and clotrimazole are involved in the treatment of various systemic fungal infections. It is a part of the theophylline molecule, present in tea leaves and coffee beans, which stimulates the central nervous system.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Imidazole is used as a buffer in the range of pH 6.2-7.8. It is also an histamine antagonist. It acts as a chelator and forms complexes with various divalent cations. It is used as a corrosion inhibitor on certain transition metals such as copper. Its derivatives, like polybenzimidazole (PBI), act as fire retardants. It finds application in photography and electronics. Imidazole salts are used as ionic liquids and precursors to stable carbenes. Imidazole derivatives like ketoconazole, miconazole and clotrimazole are involved in the treatment of various systemic fungal infections. It is a part of the theophylline molecule, present in tea leaves and coffee beans, which stimulates the central nervous system.
Solubility
Miscible with water, ethanol, ether, acetone, chloroform, pyridine and methanol. Slightly miscible with benzene and petroleum ether.
Notes
Incompatible with acids, acid anhydrides and strong oxidizing agents.
Imidazole is used as a buffer in the range of pH 6.2-7.8. It is also an histamine antagonist. It acts as a chelator and forms complexes with various divalent cations. It is used as a corrosion inhibitor on certain transition metals such as copper. Its derivatives, like polybenzimidazole (PBI), act as fire retardants. It finds application in photography and electronics. Imidazole salts are used as ionic liquids and precursors to stable carbenes. Imidazole derivatives like ketoconazole, miconazole and clotrimazole are involved in the treatment of various systemic fungal infections. It is a part of the theophylline molecule, present in tea leaves and coffee beans, which stimulates the central nervous system.
Solubility
Miscible with water, ethanol, ether, acetone, chloroform, pyridine and methanol. Slightly miscible with benzene and petroleum ether.
Notes
Incompatible with acids, acid anhydrides and strong oxidizing agents.
RUO – Research Use Only
General References:
- Nucleophilic catalyst for many acylation and silylation reactions, compare 4-(Dimethyl amino) pyridine, A13016.
- For use in: silylation of 1,3-diketones, see Hexamethyl disilazane, A15139; introduction of the TBDMS and TBDPS groups; see: tert-Butyl dimethyl chlorosilane, A13064, and tert-Butyl diphenyl chlorosilane, A12721, respectively.
- In combination with triphenylphosphine and iodine, vic-diols are converted to alkenes: Synthesis, 469 (1979), and alcohols to alkyl iodides: Synth. Commun., 20, 1473 (1990).
- With 2 moles of an aroyl halide gives, after hydrolysis, good yields of 2-aroylimidazoles: Synthesis, 675 (1978).
- Sankar, M.; Ajithkumar, T. G.; Sankar, G.; Manikandan, P. Supported imidazole as heterogeneous catalyst for the synthesis of cyclic carbonates from epoxides and CO 2. Catal. Commun. 2015, 59, 201-205.
- Hart, K. R.; Sottos, N. R.; White, S. R. Repeatable self-healing of an epoxy matrix using imidazole initiated polymerization. Polymer 2015, 67, 174-184.