Search Thermo Fisher Scientific
Thermo Scientific Chemicals
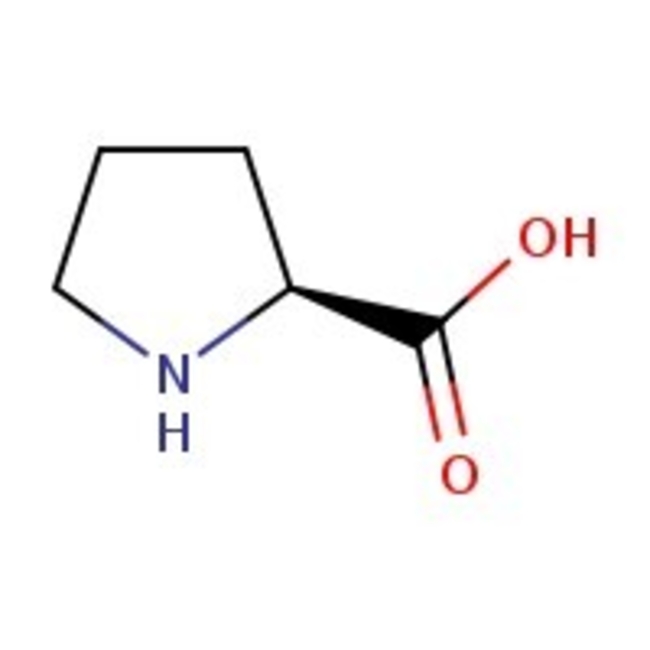
L-Proline, 99%
CAS: 147-85-3 | C5H9NO2 | 115.13 g/mol
Catalog number ALFA10199.36
Price (MYR)
2,937.00
EA
Quantity:
500 g
Price (MYR)
2,937.00
EA
Specifications
Chemical Name or MaterialL-Proline
Melting Point∼225°C (decomposition)
CAS147-85-3
Recommended StorageAmbient temperatures
RTECS NumberTW3584000
View more
L-Proline is used as asymmetric catalysts in organic synthesis and asymmetric aldol cyclization. It is involved in the Michael addition of dimethyl malonate to alfa-beta-unsaturated aldehydes. It is a precursor of hydroxyproline in collagen. It is an active component of collagen and involved in the proper functioning of joints and tendons. It finds uses in pharmaceutical, biotechnological applications due to its osmoprotectant property. Further, it is used with ninhydrin in the chromatography.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
L-Proline is used as asymmetric catalysts in organic synthesis and asymmetric aldol cyclization. It is involved in the Michael addition of dimethyl malonate to alfa-beta-unsaturated aldehydes. It is a precursor of hydroxyproline in collagen. It is an active component of collagen and involved in the proper functioning of joints and tendons. It finds uses in pharmaceutical, biotechnological applications due to its osmoprotectant property. Further, it is used with ninhydrin in the chromatography.
Solubility
Soluble in water and ethanol. Insoluble in ether, butanol and isopropanol.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
L-Proline is used as asymmetric catalysts in organic synthesis and asymmetric aldol cyclization. It is involved in the Michael addition of dimethyl malonate to alfa-beta-unsaturated aldehydes. It is a precursor of hydroxyproline in collagen. It is an active component of collagen and involved in the proper functioning of joints and tendons. It finds uses in pharmaceutical, biotechnological applications due to its osmoprotectant property. Further, it is used with ninhydrin in the chromatography.
Solubility
Soluble in water and ethanol. Insoluble in ether, butanol and isopropanol.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- For use as a catalyst in an asymmetric aldol cyclization, see: Org. Synth. Coll., 7, 363 (1990). For reaction scheme, see 2-Methyl cyclopentane-1,3-dione, A13936 . For use in a direct enantioselective crossed aldol reaction with aldehydes, see: J. Am. Chem. Soc., 124, 6798 (2002). For use as an organoctalyst in direct aldol reactions of ɑ-oxyaldehydes, as the first step in a two-step synthesis of carbohydrates, see: Angew. Chem. Int. Ed., 43, 2152 (2004). Mediates the enantioselective borane reduction of ketones to chiral alcohols with moderate to good ee: Monatsh. Chem., 131, 91 (2000). Also used to induce high ee in the ɑ-amination of aldehydes with Dibenzyl azodicarboxyl ate, L19347 : J. Am. Chem. Soc., 124, 5656 (2002), or Diethyl azodicarboxyl ate, L19348 : Angew. Chem. Int. Ed., 41, 1790 (2002).
- For a review of the use of proline as a chiral catalyst, see: Synlett, 1675 (2001); Synlett, 1973 (2006).
- For preparation from L-proline of the chiral hydrazone SAMP, and enantioselective syntheses using the RAMP/ SAMP methodology, see: Org. Synth. Coll., 8, 26, 403 (1985).
- For formation of a fused pyrrolidino-oxazolidinone, and use of the product in a synthesis of enantiopure ɑ-branched amino acids, see: Org. Synth. Coll., 9, 626 (1998); for reaction scheme, see Trimethyl acetaldehyde, A15013 .
- The Li salt has been used as a catalyst for the Michael addition of dimethyl malonate to ɑß-unsaturated aldehydes: J. Chem. Soc., Chem. Commun., 1088 (1991).
- Sferrazza, A.; Triolo, A.; M Migneco, L.; Caminiti, R. Synthesis and Small and Wide Angle X-Ray Scattering Characterization of L-Proline Based Chiral Ionic Liquids. Curr. Org. Chem. 2015, 19 (1), 99-104.
- Shimpi, M. R.; Childs, S. L.; Boström, D.; Velaga, S. P. New cocrystals of ezetimibe with l-proline and imidazole. CrystEngComm 2014, 16 (38), 8984-8993.