Search Thermo Fisher Scientific
Thermo Scientific Chemicals
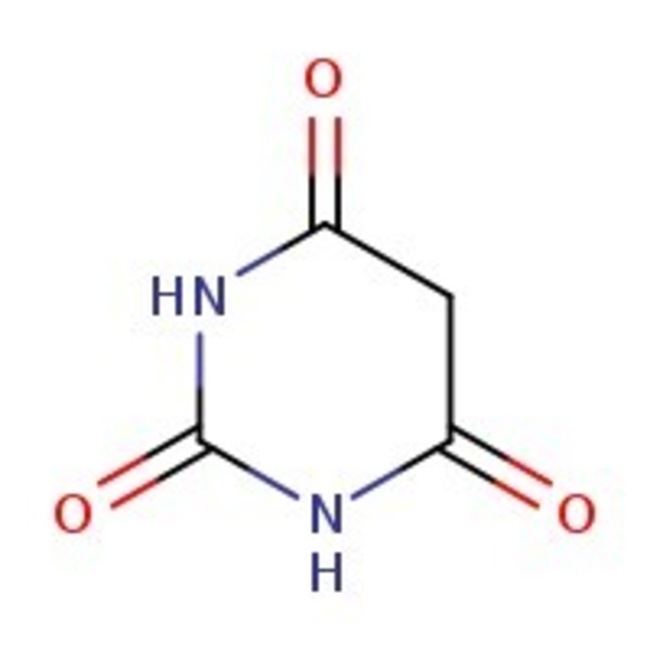
Barbituric acid, 99+%
Barbituric acid, CAS # 67-52-7, (synonym: malonylurea) is the parent compound used in the production of barbiturates that act as sedative drugs. It is also used extensively in the manufacturing of plastics, textiles, and polymers. | CAS: 67-52-7 | C4H4N2O3 | 128.09 g/mol
Catalog number ACR180925000
Price (MYR)
1,180.00
EA
Quantity:
500 g
Price (MYR)
1,180.00
EA
Specifications
Chemical Name or MaterialBarbituric acid
Name Note99+%
CAS67-52-7
Assay Percent Range99+%
Molecular FormulaC4H4N2O3
View more
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General description
• Barbituric acid is a white, odorless, water-soluble crystalline powder
• It is a strong acid in an aqueous medium
Applications
• Barbituric acid uses the Knoevenegal condensation reaction to be converted into barbiturate drugs that acts as central nervous system depressants
• It is an active ingredient in the synthesis of Vitamin B12
• It is also used in electrochemical oxidation of iodine using cyclic voltammetry and controlled potential coulometry
RUO – Research Use Only
General References:
- Ernst, B. J.; Clark, G. F.; Grundmann, O. The Physicochemical and Pharmacokinetic Relationships of Barbiturates - From the Past to the Future. Curr Pharm Des 2015, 21(25), 3681-91.
- Lingens, B.; Schild, T. A.; Vogler, B.; and Renz, B. Biosynthesis of vitamin B12. Transformation of riboflavin 2H-labeled in the 1'R position of 1'S position into 5,6-dimethylbenzimidazole Eur J Biochem
- 1992, 207(3):981-5.
- Nematollahi, D.; Hesari, M. Electrochemical study of iodide in the presence of barbituric acid. Application to coulometric titration of barbituric acid. Microchemical Journal 2001, 70(1), 7-11.